Introduction : MEZI is a novel oral cereblon E3 ligase modulator (CELMoD ™) with enhanced tumoricidal and immune-modulatory effects compared with immunomodulatory drugs (IMiDs ®). In preclinical studies, MEZI showed synergy with anti-myeloma drugs including DEX, proteasome inhibitors (PIs), and anti-CD38 monoclonal antibodies (mAbs), and is being assessed in the phase 1/2 CC-92480-MM-002 (NCT03989414) trial with different treatment combinations in RRMM. Here we report the preliminary efficacy and safety of MEZI, DARA, and DEX (MeziDd) over 3 dosing schedules, as well as MEZI, ELO and DEX (MeziEd), in pts with RRMM.
Methods : Eligible pts had: RRMM, received 2-4 prior lines of therapy, a minimal response or better to ≥ 1 prior regimen, and disease progression during or after their last therapy.
In the MeziDd cohort, oral MEZI was given at escalating doses on days (D) 1-21 per 28-D cycle (C) (subcohort B1); on D1-14 per 21-D cycle from C1-8 and D1-21 per 28-D cycle ≥ C9 (subcohort B2); or on D1-7 and D15-21 per 28-D cycle from C1-6 and D1-21 per 28-D cycle ≥ C7 (subcohort B3). Intravenous (IV; 16 mg/kg) or subcutaneous (1800 mg) DARA was given weekly (C1-2), then biweekly (C3-6), and monthly (≥ C7) for subcohorts B1 and B3, and weekly (C1-3) then on D1 of each 21-D (C4-8) or 28-D (≥ C9) cycle for subcohort B2; with weekly oral/IV DEX (40 mg; 20 mg > 75 y or body mass index < 18.5 kg/m 2).
In the MeziEd cohort, oral MEZI was given at escalating doses on D1-21 per 28-D cycle, with weekly (C1-2; 10 mg/kg) then monthly (≥ C3; 20 mg/kg) IV ELO, and oral/IV DEX on ELO dosing days (36 mg; 16 mg > 75 y) plus on D8, 15, and 22 of ≥ C3 (40 mg; 20 mg > 75 y).
Thromboembolism (TE) prophylaxis was given within 48 hours of C1D1 and until 48 hours after last MEZI dose.
Primary objectives were to determine the recommended dose and regimen and to assess safety and preliminary efficacy as overall response rate (ORR).
Results : As of the May 25, 2023 data cut, 57 pts were enrolled and had received MeziDd (B1: n = 23; B2: n = 16; B3: n = 18) at the 0.3 mg or 0.6 mg dose. Median age was 67 (range, 45-83) y, median time since initial diagnosis was 8.2 (1.0-15.8) y, and median number of prior regimens was 2 (2-5). Prior therapies included stem cell transplantation (15.8%), IMiD agents (100%), PIs (98.2%), and anti-CD38 mAbs (8.8%); 82.5% of pts were refractory to IMiD agents and 61.4% to PIs. Extramedullary plasmacytomas were present in 4 (7.0%) pts.
Forty-five of 57 (78.9%) pts continued treatment; the main reason for discontinuation was progressive disease (5 pts; 8.8%).
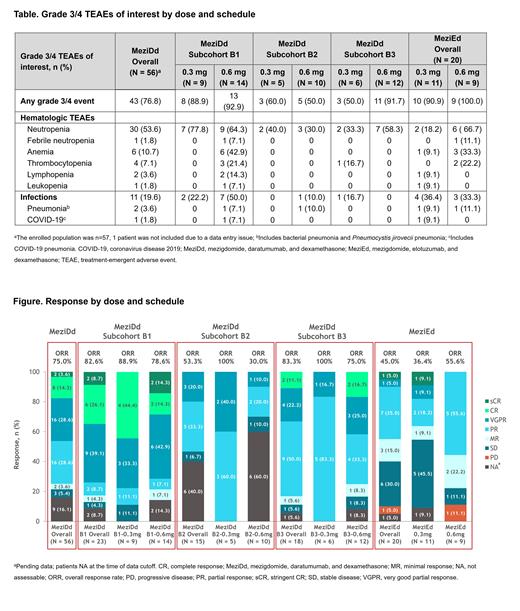
In the safety population (n = 56), ORR was 75.0% overall, with 2 (3.6%) stringent complete responses, 8 (14.3%) complete responses, 16 (28.6%) very good partial responses, and 16 (28.6%) partial responses (Figure). Duration of response and progression-free survival data were not yet mature.
Grade (Gr) 3/4 treatment-emergent adverse events (TEAEs) occurred in 43/56 (76.8%) pts and were mostly hematologic (Table). Gr 3/4 neutropenia occurred in 30 (53.6%) pts; Gr 3/4 thrombocytopenia occurred in 4 (7.1%) pts (3/4 pts were in subcohort B1-0.6 mg) and was not associated with bleeding. Gr 3/4 infections occurred in 11 (19.6%) pts and proved manageable in all cases (Table). Non-hematologic Gr 3/4 TEAEs were low. No Gr 3/4 TE or peripheral neuropathy was observed. MEZI dose reductions were needed for 7 (30.4%) pts in subcohort B1 (1 at 0.3 mg, 6 at 0.6 mg) and 1 (5.6%) pt in subcohort B3 (at 0.6 mg). In the evaluable population (n = 35), 5 (14.3%) pts had ≥ 1 dose-limiting toxicity. The maximum tolerated dose was not reached. MeziDd had pharmacodynamically active immune stimulation in T and NK cells in all 3 schedules and both doses.
Twenty pts received MeziEd at 0.3 mg (n = 11) or 0.6 mg (n = 9); median prior lines of therapy was 3 (2-4) and 3 (2-5), and most pts (72.7% and 100%) had prior anti-CD38 mAb exposure. ORRs were 36% and 56%, respectively. The safety profile was consistent with known TEAEs and was generally well tolerated, although 2 Gr 3 pulmonary embolisms occurred.
Conclusions : MeziDd showed promising efficacy and a manageable safety profile in pts with RRMM and 2-4 prior lines of therapy, as did MeziEd in pts with prior anti-CD38 mAb therapy. The immune activity of MEZI was consistent with previous preclinical reports. Improved safety and efficacy may be achieved by schedule and dose adjustments. These results support further evaluation of MEZI plus anti-myeloma mAbs in phase 1/2 and phase 3 studies. Updated results will be presented at the meeting.
Disclosures
Richardson:Oncopeptides: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Other: Contracted research, Research Funding; GSK: Consultancy; Takeda: Research Funding; Sanofi: Consultancy; AstraZeneca Pharmaceuticals LP, Bristol-Myers, Squibb Company, Celgene Corporation, GlaxoSmithKline, Janssen Biotech Inc, Karyopharm Therapeutics, Oncopeptides, Sanofi, Secura Bio, Takeda Pharmaceuticals USA Inc;: Consultancy; Karyopharm: Consultancy, Research Funding. Sandhu:Celgene/BMS: Honoraria; Pfizer: Honoraria; Gilead/Kite: Honoraria; Forus: Honoraria; Janssen: Honoraria; Sanofi: Honoraria. Hofmeister:Pfizer: Research Funding; Sanofi: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding. Orlowski:Asylia Therapeutics, BioTheryX Inc., Heidelberg Pharma: Other: Laboratory Research Funding, Research Funding; BMS/Celgene Corporation, CARsgen Therapeutics, Exelixis Inc., Heidelberg Pharma, Janssen Biotech Inc., Sanofi/Genzyme, Takeda Pharmaceuticals USA Inc.: Other: Clinical Research Funding, Research Funding; AbbVie, Adaptive Biotech, Asylia Therapeutics, Inc., BioTheryX, Bristol-Myers Squibb Pharmaceuticals, Karyopharm Therapeutics, Meridian Therapeutics, Monte Rosa Therapeutics, Nanjing IASO Biotherapeutics, Neoleukin Corporation, Oncopeptides AB, Pfizer, In: Consultancy, Honoraria; Asylia Therapeutics: Current equity holder in private company, Patents & Royalties. White:Amgen: Consultancy, Honoraria; Antengene: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Forus: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Belotti:Pfizer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Raje:Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Immuneel: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Caribou Biosciences: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; K36 Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Chow:BMS: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Zhou:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Civardi:Celgene International Sàrl, a Bristol-Myers Squibb Company,: Current Employment. Koo:Alexion: Other: Spouse is an employee and equity holder; Novartis: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Zhu:Bristol Myers Squibb: Current Employment. Katz:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Oriol:GSK: Consultancy, Honoraria, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Other: Consulting fees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal